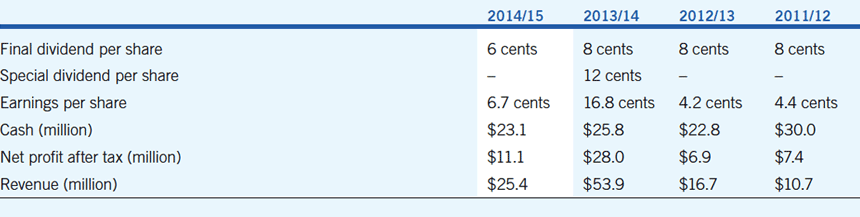
- Earnings per share 6.7 cents, facilitating a final dividend payment of 6 cents per share, fully-franked
- Board-endorsed strategy with a clear focus on diversifying our product pipeline and creating future growth opportunities utilising the Acrux technology platform
- Good progress on our onychomycosis program, targeting a once daily treatment with a superior drug permeation profile
- Topical and transdermal generics pipeline identified and development initiated
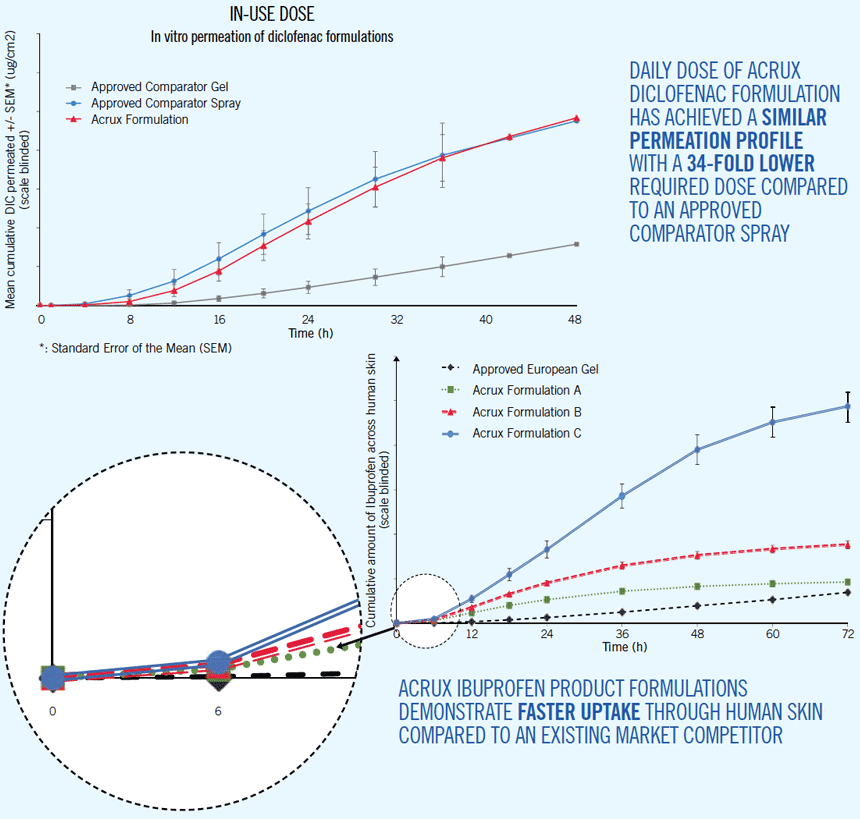
- Superior permeation profiles demonstrated with formulation of non-steroidal anti-inflammatory drugs (NSAID), compared to market leaders
- Acrux net profit after tax (NPAT) of $11.1 million (2014: $28.0 million). Profit in the prior year was higher as milestone payments were received in 2014
- Royalty income of $24.6 million (2014: $24.7 million). Declines in the sales volumes of Axiron were largely offset by improvements in the AUD$:USD$ exchange rate
- Cash reserves: $23.1 million (2014: $25.8 million) after payment of dividends in the current and prior financial years. Acrux does not have any debt facilities
- Regulatory approval for Lenzetto (estradiol spray) granted in Europe
- Launch of Lenzetto in Europe, Acrux’s unique estradiol spray for treating post-menopausal symptoms which is licensed exclusively in Europe to Gedeon Richter
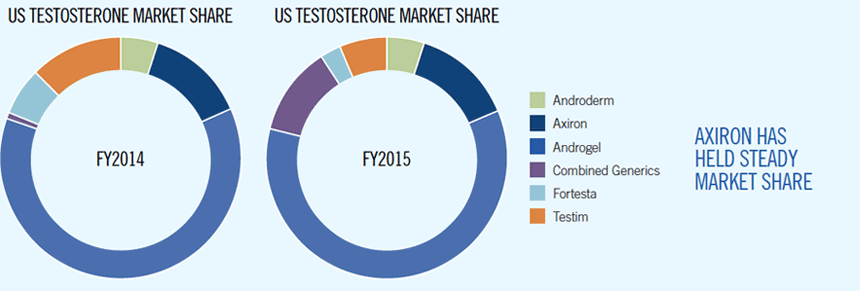
- 2016 National Formulary status for Axiron has been secured at two of the largest commercial prescription benefit managers (PBMs)
- Axiron anticipated to maintain market share during the 2016 financial year, with stabilisation of prescription volumes over the first half 2015 calendar year
- Progression of development programs for multiple product candidates with no material change to ongoing cash operating cost needs (until one or more projects enters into clinical trials)