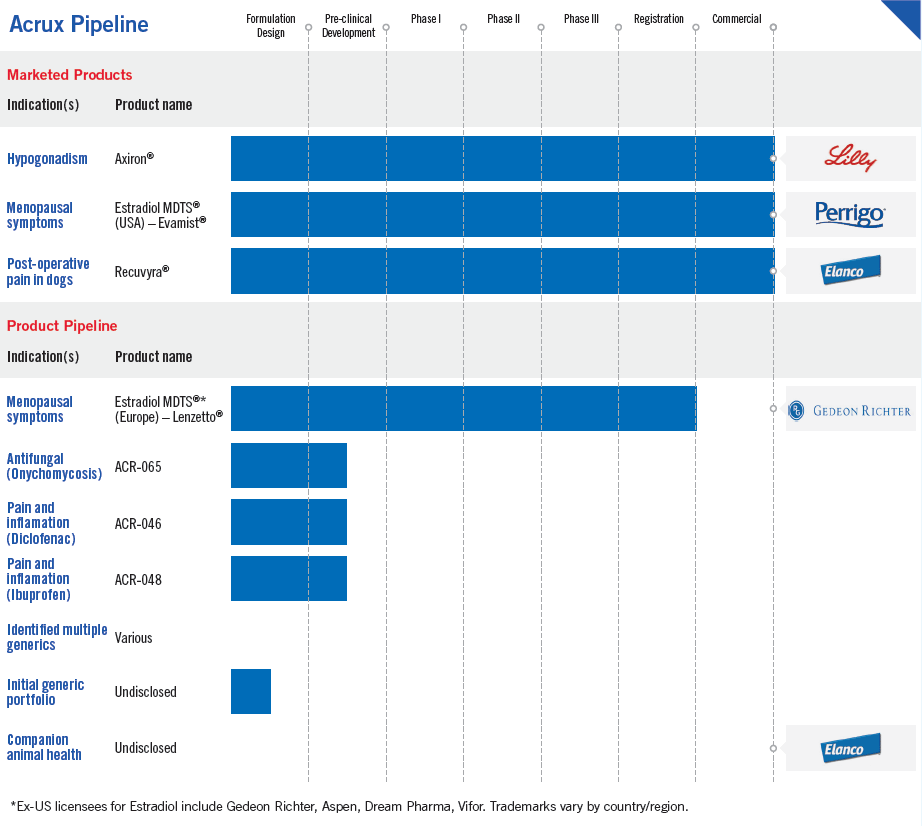
ACRUX IS BUILDING A DIVERSIFIED PRODUCT PORTFOLIO BASED ON MAKING CAPITAL RETURNS, MAINTAINING SUSTAINABLE MARKET ADVANTAGES AND PROVIDING CLEAR BENEFITS TO PATIENTS, PHYSICIANS AND HEALTHCARE PAYERS.
Onychomycosis
Acrux continues to develop a topical antifungal product for the treatment of onychomycosis (fungal infection in fingernails and toenails). It is anticipated that the product will be a once daily application that provides better efficacy than the current lead comparator product. In parallel with ongoing preclinical studies, the clinical trial design and regulatory pathway for the product are being planned.
Dispute with Hexima
Our dispute with Hexima over the antifungal collaboration agreement remains formally unresolved. This does not affect our current onychomycosis program.
Pain
Acrux’s proprietary topical NSAID products for pain have completed formulation design.
Diclofenac
Based on preclinical studies, the daily dose of Acrux Diclofenac formulation has achieved a similar permeation profile with a 34-fold lower dose requirement compared to an approved comparator spray. In addition, the permeation profile is such that the dose would support a once daily application. Current competitors’ products require at least a twice daily application to maintain the drug delivery profile. This differential feature provides a significant improvement over existing products.
Ibuprofen
Early studies indicate that Acrux’s ibuprofen product formulations demonstrate a significantly faster uptake through human skin relative to existing competitors, which would result in a much faster onset of pain relief. Our product delivery profile also supports a once daily application, providing a significant improvement over currently approved products.
With both of these NSAID opportunities, Acrux intends to work closely with prospective corporate partners that are both capable and committed to further development and marketing in key territories.
Generics
The first wave of topical and transdermal generic product opportunities has been identified and product development has been initiated. The local market value of the initial 12 products identified has market sales of US$2.4 billion in countries we are targeting. For competitive reasons the pipeline remains undisclosed.
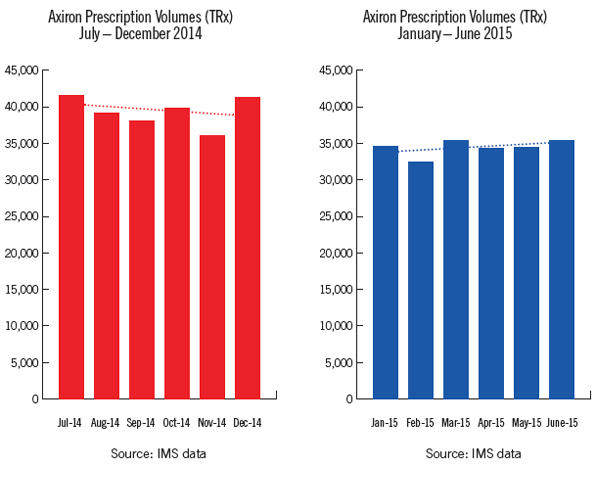
Axiron
IMS market share for lead testosterone products

Axiron TRx (prescription volume) for financial year 2014/15
THE 2016 NATIONAL FORMULARY STATUS FOR AXIRON HAS BEEN SECURED AT TWO OF THE LARGEST COMMERCIAL PRESCRIPTION BENEFIT MANAGERS (PBM).
Axiron
FDA Update In March 2015, the Food and Drug Administration released a Communication regarding the use of Testosterone Replacement Therapy in the US, titled “FDA Cautions About Using Testosterone Products for Low Testosterone Due to Aging; Requires Labeling Change to Inform of Possible Increased Risk of Heart Attack And Stroke”. These class labelling changes were implemented for all testosterone products in June 2015. Sponsors of marketed testosterone products in the United States are in dialogue with the FDA over a long term safety trial. The protocols for this are being planned with finalisation expected in mid-2016.
Axiron EMA Update
In November 2014, the European Medicines Agency (EMA) released a statement regarding the use of Testosterone Replacement Therapy in Europe, titled: “No consistent evidence of an increased risk of heart problems with testosterone medicines”. The committee considered that the benefits of testosterone continue to outweigh its risks but recommended that testosterone-containing medicine should only be used where lack of testosterone has been confirmed by signs and symptoms as well as laboratory tests. Labelling changes were not required for Axiron in Europe.
Axiron Litigation
The Acrux patent concerning the underarm administration of testosterone formulations was granted by the United States Patent and Trademark Office (USPTO) in May 2013. In the United States, Axiron is now protected by a number of patents which have expiry dates of 2030, 2027, 2026 and 2017. The patents cover 3 different aspects of the product, including the formulation and delivery method, administration to the underarm and the physical applicator.
In May 2013, November 2013, December 2014 and July 2015, Acrux DDS Pty Ltd (a wholly-owned subsidiary of Acrux Ltd.) together with Eli Lilly and Company (“Lilly”) filed lawsuits in the United States District Court for the Southern District of Indiana against 1) Perrigo Israel Pharmaceuticals Limited, 2) Watson Laboratories Inc. (“Activis”), 3) Amneal Pharmaceuticals LLC and 4) Lupin Pharmaceuticals Inc., respectively for infringement of issued patents covering Axiron. In each instance, the patents are owned by Acrux DDS and are exclusively licensed to Lilly. The lawsuits were filed in response to notice letters sent by each company regarding its filing with the United States Food and Drug Administration of an Abbreviated New Drug Application (ANDA) for a Testosterone Metered Dose Transdermal Solution. The letters each stated that the respective ANDAs contain Paragraph IV certifications with respect to United States patents that include claims relating to the application of testosterone formulations to the underarm and to the applicator used to apply Axiron. Activis further includes paragraph IV certifications with respect to United States patents that include claims relating to the quick-drying formulation. A Paragraph IV certification alleges invalidity, unenforceability and/or noninfringement of a patent.
Throughout 2014, a number of pending product liability lawsuits were filed against Acrux and Lilly in the United States District Court for the Northern District of Illinois, including claims that assert injury caused by testosterone replacement therapy. These cases, brought by private plaintiffs, were consolidated for pretrial purposes in the United States District Court for the Northern District of Illinois under the Multi-District Litigation Rules as Testosterone Replacement Therapy Products Liability Litigation, MDL No. 2545.
Estradiol Spray
The first product developed by Acrux was an estradiol spray for women to treat the symptoms of menopause. The spray was approved by the FDA in 2007 and launched into the US market in 2008. Branded Evamist, the spray is now distributed in the United States by Perrigo Company plc. Evamist US net sales are currently approximately US$10 million per annum.
In June 2013, Acrux appointed Gedeon Richter to commercialise the product in certain ex-US markets and received US$1 million upon signing the agreement. In September 2015, Gedeon Richter and Acrux announced that the product, named Lenzetto, received multiple regulatory approvals in European territories, triggering milestone payments totaling US$2 million. These approvals of Lenzetto were granted after the European decentralised procedure (DCP) was completed with the first country approval triggering a milestone payment of US$ 1 million and the second and third approvals triggering milestone payments of US$ 0.5 million each. Initial launches are planned for the first half of calendar year 2016 following pricing and reimbursement approvals.
ACRUX GENERATES INCOME FROM COMMERCIALISED PRODUCTS, IS CASHFLOW POSITIVE AND MAINTAINS A LOW OPERATING COST BASE.
Revenue
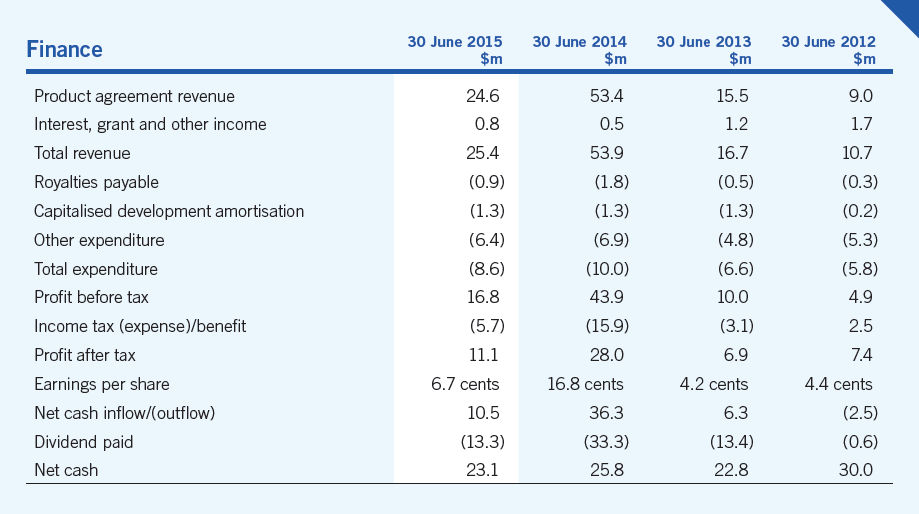
Total revenue for the financial year was $25.4 million (2014: $53.9 million). Revenue from product agreements was $24.6 million (2014: $53.4 million). No milestones were recognised during the year (2014: $28.7 million). The prior year revenue included two one-off milestones - firstly, $28.0 million (US$25 million) from Lilly for Axiron sales exceeding US$100 million during the 2014 calendar year and secondly, a $0.7 million (US$0.6 million) milestone from Gedeon Richter following the first European regulatory filing for Acrux’s estradiol spray in Europe. Interest income contributed $0.6 million (2014: $0.5 million).
Expenses
Operating expenditure decreased to $8.6 million (2014: $10.0 million). Royalty payments due to Monash Investment Trust decreased to $0.9 million (2014: $1.8 million), in line with decreased product income. The continued strength of the US dollar against the Australian dollar resulted in a favourable conversion of product income, which is received in US dollars. No foreign exchange losses were recorded during the financial year (2014: $1.2 million). Directors’ fees decreased to $0.4 million (2014: $0.6 million) while employee benefits expense increased to $2.7 million (2014: $2.3 million). Professional fees increased to $0.7 million (2014: $0.3 million) as a result of investment in strategic advice, coupled with legal costs incurred in the dispute with Hexima.
A non-cash expense of $0.8 million (2014: $0.6 million) was recorded for employee share options granted during the reporting period, as required by accounting standard AASB 2. Income tax expense for the financial year was $5.7 million (2014: $15.9 million) representing approximately 33.8% of profit before income tax. Acrux Limited is a Pooled Development Fund (PDF). The income tax expense recorded is the result of the tax effect particular to a PDF. PDFs are taxed at 15% on operating income. Subsidiaries of a PDF are taxed at 30% on operating income. Groups containing a PDF are not permitted to consolidate for tax purposes. Further information regarding income tax expense is provided at Note 1(j) of the notes to the financial statements following.
Cash flow
Cash reserves at the end of the financial year were $23.1 million (2014: $25.8 million). Net cash outflow over the period totaled $2.9 million (2014: a net cash inflow of $3.0 million was recorded).
The outflow of cash recorded for financing activities represents the payment of $13.3 million (2014: $33.3 million) of dividends to shareholders, representing the 8 cent final dividend for the 2013/14 financial year.
Cash receipts from product agreements decreased to $25.2 million (2014: $53.4 million) with no milestones received. Interest receipts added $0.6 million (2014: $0.5 million). Payments to suppliers and employees decreased to $6.5 million (2014: $6.7 million). Income taxes paid decreased to $8.9 million (2014: $10.8 million).
Outlook
Acrux generates income from commercialised products, is cashflow positive and maintains a low operating cost base.
Operating cost base
Acrux will continue to progress its product pipeline. The annual cash operating expenditure to execute our preclinical program is expected to remain consistent with the 2015 financial year, being $5.5 million. This excludes Monash royalty payments and non-cash items. This expenditure covers maintenance of the Company’s operations, research and development, and intellectual property expenses. Royalty payments due to Monash Investment Trust cease in February 2017. The expenditure profile of the business will naturally increase as projects enter into clinical trials. Acrux will update the market accordingly prior to this occurring.
Royalties
In the short term, Acrux will derive the majority of its revenue under its global license agreement with Lilly for the marketing and distribution of Axiron. Royalty income will increase from our estradiol products. In the US, Perrigo has been appointed as the distributor through Perrigo’s women’s health franchise. As noted previously, Acrux announced the approval of Lenzetto, Acrux’s estradiol spray for the relief of menopausal symptoms for women, licensed exclusively to Gedeon Richter in Europe. Launch in Europe is expected in the first half of calendar 2016.
Milestones
Under our agreements, Acrux may also be eligible to receive milestones for defined product achievements. During the quarter ended September 2015, Acrux announced that a US$2 million milestone was earned upon receipt of regulatory approvals for Lenzetto in Europe. Sales of Axiron are not anticipated to trigger a sales based milestone during 2016, however a revenue based milestone payment of US$50 million is payable upon Axiron achieving an undisclosed global sales hurdle. Acrux may be eligible for further milestone payments totaling US$120 million over the four financial years commencing 2018/19.
Risk and uncertainty
Forward-looking statements are subject to risks and uncertainties and have been made throughout this report. Such statements involve known and unknown risks and important factors that may cause the actual results, performance or achievements of Acrux to be materially different from statements made in this report.